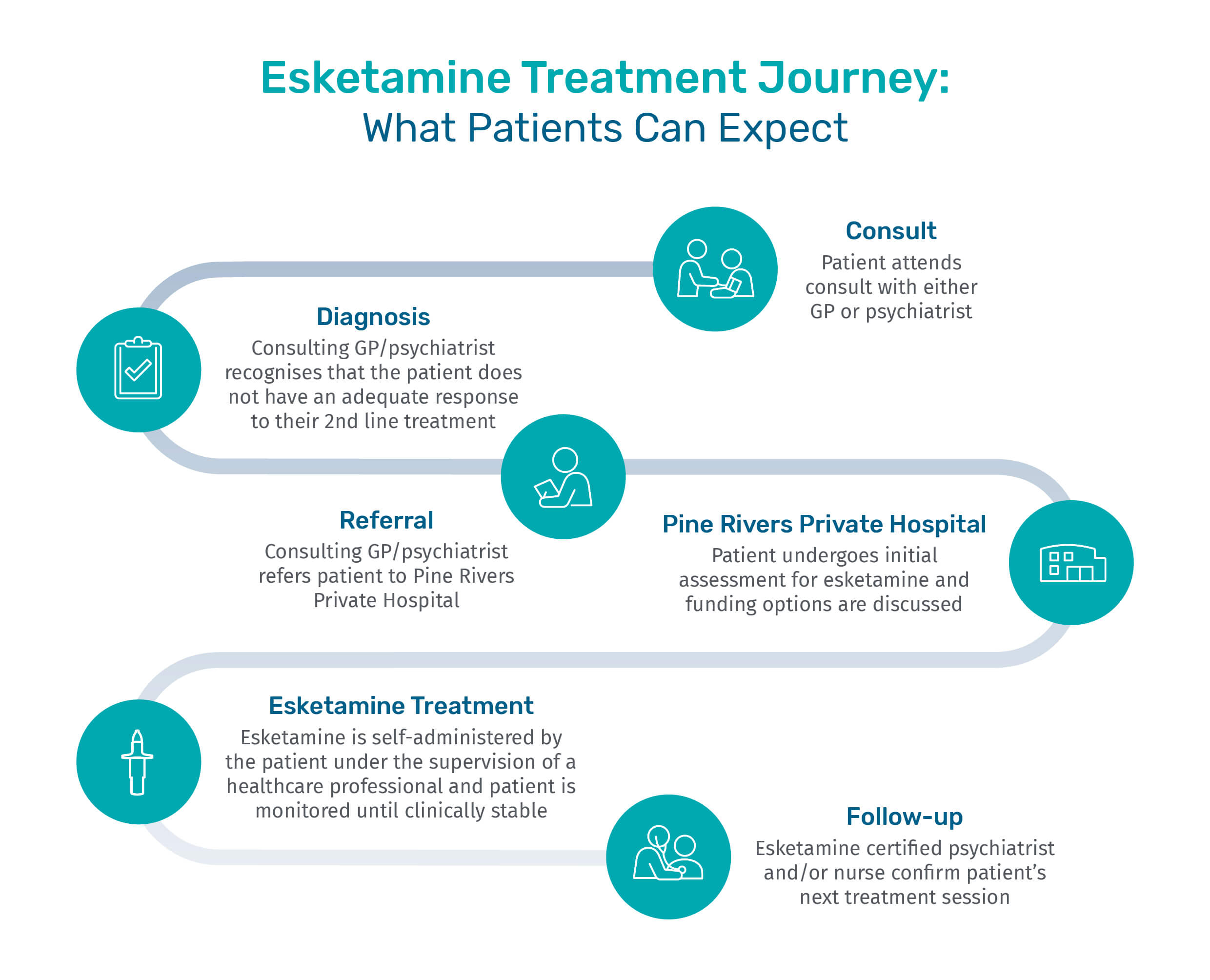
Eligibility for esketamine treatment
If you have trialled two or more oral antidepressant medications in your current episode of depression and continue to experience symptoms of depression, you may be eligible for esketamine treatment.


Esketamine is indicated for treatment-resistant depression – this is defined as Major Depressive Disorder in adults who have not responded adequately to at least two different antidepressants of adequate dose and duration to treat the current moderate to severe depressive episode. [1]
Esketamine nasal spray (marketed as Spravato®) is a Schedule 8 controlled medicine that is included in the Australian Register of Therapeutic Goods (ARTG) for authorised supply in Australia.
It can only be administered at approved treatment centres like Pine Rivers Private Hospital, where clinicians have completed the necessary training and meet strict regulatory and safety standards.
The nasal spray is designed for self-administration by the patient, but only under the direct supervision of a qualified healthcare professional in a clinical setting. [2]
If you have trialled two or more oral antidepressant medications in your current episode of depression and continue to experience symptoms of depression, you may be eligible for esketamine treatment.

Please speak with your GP about arranging a referral to a psychiatrist at Pine Rivers Private Hospital.
To ensure your referral is directed to a psychiatrist trained in esketamine treatment, your GP must specify in the referral that it is for consideration of esketamine. This will help ensure you are matched with a credentialed specialist who can assess your suitability for this treatment.

Patients who are eligible for treatment will undergo the following treatment phases;
Esketamine is to be initiated in conjunction with a newly initiated oral antidepressant [2].
Qualified medical and nursing staff will monitor you during the administration of the intranasal medication, and for two hours following administration. Patients are not permitted to drive home following treatment sessions, nor drive for a 24-hour period post-treatment.
Pine Rivers Private Hospital supports patients seeking esketamine treatment, offering both Inpatient (hospital-stay) and Outpatient (community-based) options.
All treatment pathways are discussed in detail to ensure the most suitable approach is chosen based on individual circumstances. Your psychiatrist will work with you to determine the best option tailored to your needs.

As of May 1st, 2025, Esketamine is subsidised under the Pharmaceutical Benefits Scheme (PBS) in Australia for patients with treatment-resistant depression (TRD).
The Australian Government, through the PBS, now covers a significant portion of the cost of treatment. This means eligible patients pay only a reduced co-payment, similar to other PBS-listed medications.
To access the PBS subsidy, patients must:
Self-funding is also available. Some Private Health Insurers or other organisations such as DVA, WorkCover or ADF may cover some of the cost of this treatment.
We recommend contacting our Admissions team to discuss your individual circumstances and funding options available to you.

Phone: 07 3881 7291
Referrals can be sent to:
Email: Pinerivers.referral@healthscope.com.au
Fax: 07 3881 7545
[1] The SPRAVATO® trademark and brand name are the property of Johnson & Johnson, its affiliates or third-party owners.
[2] Esketamine nasal spray (marketed as Spravato) is a prescription-only medicine used for treatment-resistant depression. It is administered under medical supervision. Possible side effects include dissociation, sedation, and increased blood pressure. Patients must be monitored after each dose. Please consult the Consumer Medicines Information (CMI) and speak with your doctor to see if this treatment is suitable for you.